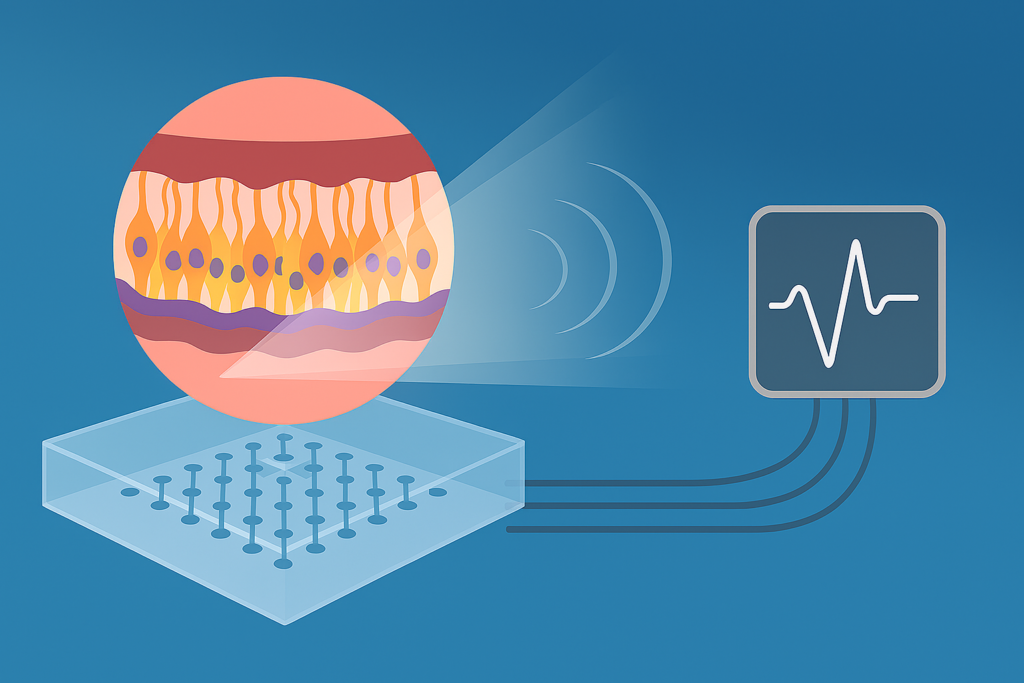
Researchers at Radboud University Medical Center, in collaboration with the University of Twente, have developed a retina-on-chip platform that combines patient-derived retinal organoids with microelectrode arrays to evaluate the functional impact of gene therapies for rare inherited eye diseases. These conditions often lead to progressive vision loss and are difficult to treat due to the complexity of retinal structure and the limited availability of human tissue for testing. The new system allows scientists to measure electrical responses to light stimuli in lab-grown retinal tissue, providing insight into whether experimental therapies restore visual signal transmission.
Retinal organoids are three-dimensional cell cultures that mimic the layered architecture of the human retina. By growing these organoids from induced pluripotent stem cells, researchers can create patient-specific models of disease. The microelectrode arrays record electrical activity across multiple points, enabling detailed analysis of how light signals are processed. This setup supports functional testing that goes beyond molecular or structural assessment, offering a more complete view of therapeutic efficacy.
The retina-on-chip project expands this approach by integrating all three layers of the retina—the choroid, pigment epithelium, and nerve layer—into a microfluidic chip that replicates the retina’s thickness and internal interactions. These chips allow researchers to monitor how light, pressure, blood flow, and mechanical stimuli affect retinal function in a controlled environment. The platform also enables precise manipulation of these parameters to study disease mechanisms and treatment effects. Researchers emphasize that this level of structural and functional integration has not been achieved in previous models.
The system is designed to support high-throughput testing, with efforts underway to reduce variability and scale production. Challenges remain, including the time required to reprogram stem cells and the difficulty of consistently incorporating the nerve layer. Despite these hurdles, the team has produced prototypes that demonstrate measurable responses linked to specific eye diseases. The platform is being used to screen gene therapies targeting known mutations and to assess long-term safety and durability.
This technology could accelerate the development of personalized treatments by allowing preclinical testing on patient-derived tissue before moving to clinical trials. It also addresses a major gap in ophthalmic research, where animal models often fail to capture the complexity of human retinal disease. The researchers believe the platform may be adapted for other neurodegenerative conditions involving sensory tissue, expanding its impact beyond ophthalmology.
By combining stem cell biology, bioengineering, and clinical expertise, the retina-on-chip platform represents a significant step toward functional, scalable, and patient-relevant testing of therapies for rare eye diseases. The work reflects a broader commitment to translating fundamental research into tools that support real-world clinical innovation.
Article from Radboud UMC: New technology offers promising perspectives for rare eye diseases