A new retina-on-a-chip platform is offering researchers a powerful tool to study retinal vein occlusion, one of the leading causes of blindness worldwide. Developed by a multidisciplinary team from POSTECH in Korea, the model mimics the complex vascular and neural environment of the human retina, enabling real-time observation of disease progression and drug response.
Retinal vein occlusion (RVO) is often triggered by chronic conditions such as hypertension and diabetes. When a retinal vein becomes blocked, it leads to fluid buildup, inflammation, and abnormal blood vessel growth, ultimately resulting in irreversible vision loss. Current treatments like anti-VEGF injections and laser therapy can slow progression but do not fully restore damaged tissue. One major barrier to developing better therapies has been the lack of physiologically accurate disease models.
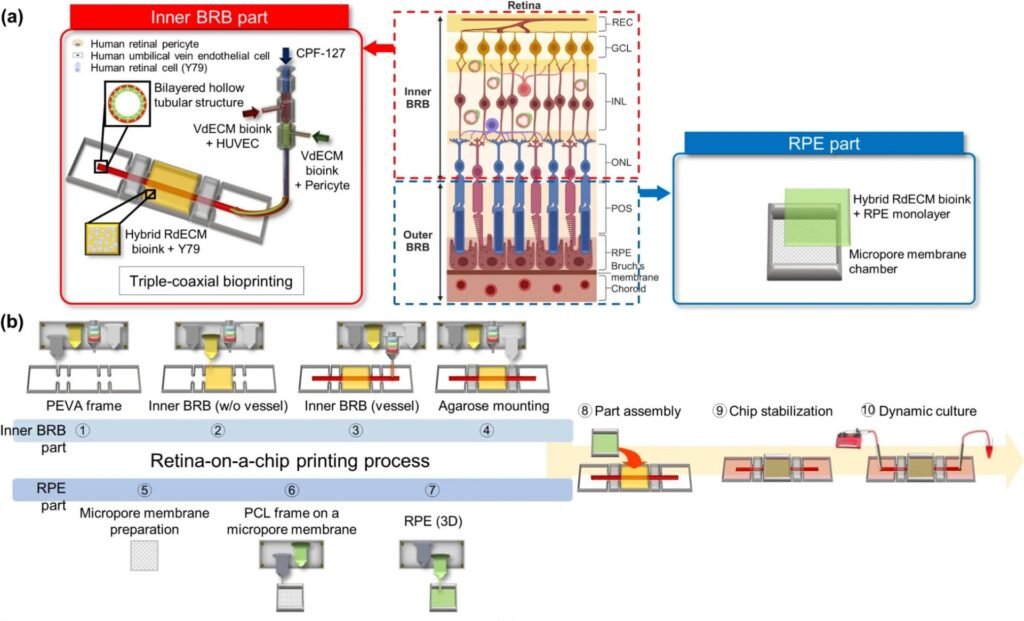
To address this, the research team created a 3D-bioprinted retina-on-a-chip using a hybrid bioink made from retinal decellularized extracellular matrix (RdECM). This bioink was used to fabricate layered structures that include both vascular and neural components. By inducing vascular occlusion within the chip, the model successfully reproduced key features of RVO, including inflammation, barrier dysfunction, and pathological angiogenesis. The system allowed researchers to observe how endothelial and retinal cells interact under disease conditions.
Drug testing on the model showed strong alignment with clinical outcomes. Aspirin suppressed vascular damage, while dexamethasone and bevacizumab reduced inflammation and abnormal vessel growth. These results confirm the model’s ability to replicate pharmacological responses seen in actual patients, making it a promising platform for preclinical drug evaluation and personalized treatment screening.
Press Release: Conquering intractable blindness with an artificial retina
Abstract in Advanced Composites and Hybrid Materials: Development of a 3D cell-printed RVO model by advancing a retina-on-a-chip with hybrid retinal dECM bioink and an integrated 3D bioprinting system