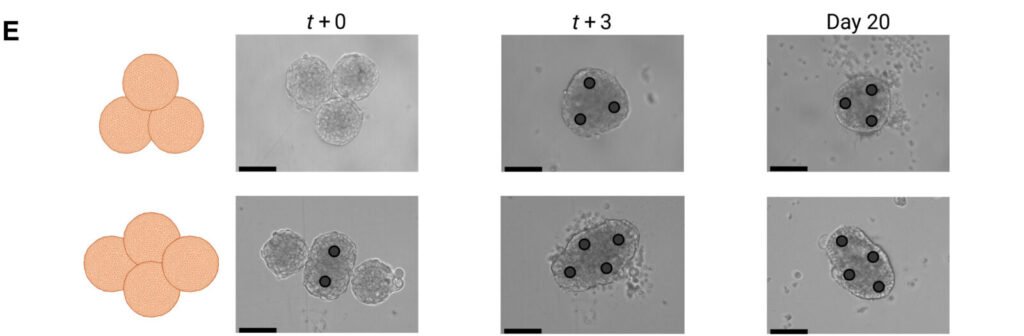
Tiny hair-like structures called cilia play a critical role in how fluids move across surfaces in the human body, from clearing mucus in the lungs to circulating cerebrospinal fluid in the brain. Understanding how cilia work has been difficult due to their microscopic size and complex behavior. Researchers at Carnegie Mellon University have developed a new robotic platform called CiliaBot that mimics the motion of cilia, allowing scientists to study their dynamics in greater detail.
CiliaBot is a modular system made up of flexible, motorized filaments that can be programmed to move in coordinated patterns. These artificial cilia are designed to replicate the beating motion of biological cilia, including the ability to synchronize and generate fluid flow. The platform allows researchers to test how different motion patterns affect fluid movement, helping them better understand the physics behind natural cilia behavior.
The team, led by Professor Carmel Majidi from the Department of Mechanical Engineering, created CiliaBot to bridge the gap between biological observation and physical modeling. By controlling the motion of each filament individually or in groups, researchers can simulate a wide range of cilia behaviors. This includes metachronal waves, where cilia beat in a coordinated sequence to move fluid efficiently.
One of the key advantages of CiliaBot is its scalability. The system can be expanded to include more filaments or adjusted to test different fluid environments. This flexibility makes it useful not only for studying biological systems but also for designing microfluidic devices and soft robots that rely on similar principles of motion and control.
The researchers believe that CiliaBot could lead to new insights into diseases linked to cilia dysfunction, such as respiratory illnesses and hydrocephalus. It may also inspire new technologies in biomedical engineering, including targeted drug delivery systems and artificial organs that mimic natural fluid transport.
Article from CMU: Designer biobots made from human lung cells
Abstract in Science Advances: AggreBots: Configuring CiliaBots through guided, modular tissue aggregation