Researchers at Linköping University in Sweden have developed a promising new approach to treating severe burns and wounds using a living gel that can be injected or 3D-printed directly into damaged skin. Dubbed “skin in a syringe,” the technology combines regenerative medicine and materials science to create a cell-rich gel that mimics the deeper layers of skin—offering hope for healing without the scarring that often follows traditional skin grafts.
Severe burns typically destroy both the outer layer of skin, known as the epidermis, and the deeper dermis, which contains blood vessels, nerves, and hair follicles. While current treatments often involve transplanting a thin layer of epidermis, this approach rarely restores full skin function and often leads to stiff, scarred tissue. Transplanting the dermis is not usually feasible, as harvesting it causes wounds as large as the ones being treated.
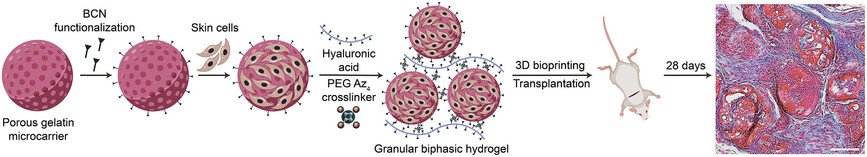
To overcome this challenge, the Linköping team focused on creating a material that could deliver the building blocks of dermal tissue directly into the wound. The key ingredient is fibroblasts—connective tissue cells that are abundant in the dermis and capable of developing into more specialized cell types. These cells were grown on tiny, porous beads made of gelatin, a substance similar to collagen found in skin. The beads provide a scaffold that helps the cells survive and organize.
However, simply pouring these beads onto a wound wouldn’t work—they would wash away or fail to stay in place. To solve this, the researchers mixed the beads with a gel made from hyaluronic acid, a naturally occurring substance in the body that helps retain moisture and support tissue repair. Using a chemical process called click chemistry, the beads and gel were linked together to form a shear-thinning material. This means the gel becomes liquid under pressure—such as when pushed through a syringe or printer nozzle—but solidifies once applied to the wound.
In laboratory tests and mouse studies, the gel showed remarkable potential. The fibroblasts remained alive and active for weeks after application, producing key proteins like collagen and laminin that are essential for forming functional skin. The implants also developed new blood vessels, which are critical for long-term tissue survival and integration. Compared to cell-free versions, the living gel grafts remodeled faster and integrated more effectively with surrounding tissue.
The researchers believe this technology could eventually allow doctors to grow a patient’s own skin cells from a small biopsy, mix them into the gel, and print or inject the material directly onto a wound. This would eliminate the need for donor skin and reduce the risk of rejection or scarring. The team also developed related hydrogel threads that can form tiny tubes for growing blood vessel cells, potentially advancing organoid development and engineered tissue systems.
Article from Linköping University: “Skin in a syringe” a step towards a new way to heal burns
Abstract in Advanced Healthcare Materials: Biphasic Granular Bioinks for Biofabrication of High Cell Density Constructs for Dermal Regeneration