Researchers at Florida Atlantic University (FAU) have developed a sophisticated computer model that maps how blood flows through the brain’s smallest and most elusive vessels. This work offers new insights into how the brain protects itself from damage and maintains a steady supply of oxygen and nutrients—especially during changes in blood pressure or bursts of neural activity. The findings, published in PLOS ONE, could help improve diagnosis and treatment of conditions like stroke, Alzheimer’s disease, and traumatic brain injury.
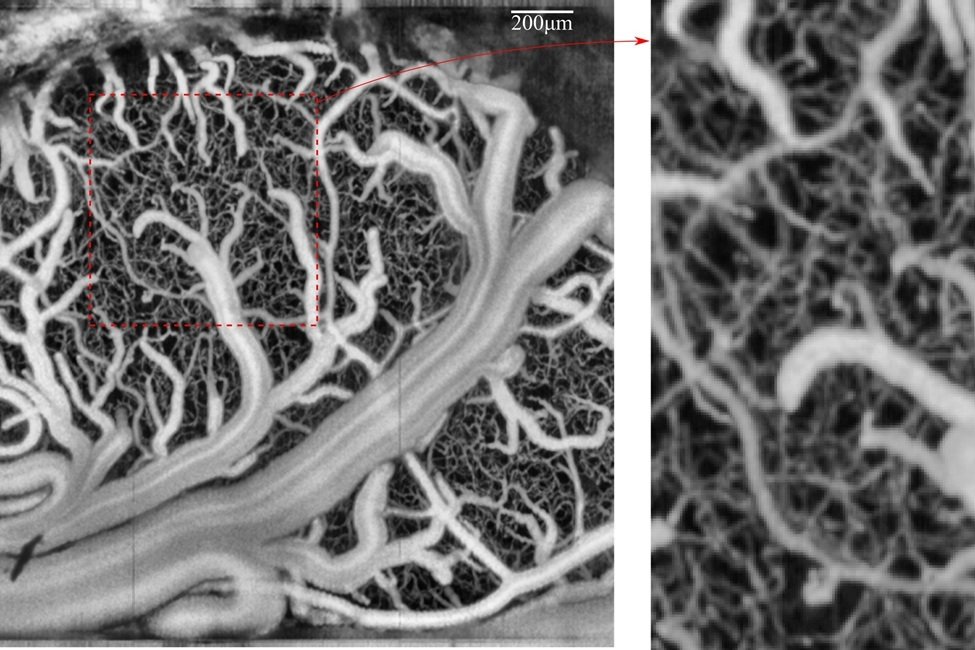
The brain relies on a vast network of blood vessels, ranging from large arteries to microscopic capillaries. Between these extremes lie transitional zone (TZ) vessels, such as penetrating arterioles, precapillary arterioles, and capillary sphincters. These vessels act as critical intermediaries, adjusting blood flow as needed to meet the brain’s metabolic demands. Despite their importance, the exact role of TZ vessels has remained unclear—especially during functional hyperemia, the process by which blood flow increases in response to brain activity.
To explore these dynamics, FAU engineers and scientists from the Sensing Institute (I-SENSE) created a detailed simulation of the mouse brain’s vascular system. Each vessel segment in the model behaves like a tiny valve, capable of adjusting its shape and flow resistance in response to changing conditions. The model integrates two key processes: hemodynamics, which describes how blood moves through vessels, and vasodynamics, which captures how vessels actively change shape to regulate that flow.
By combining these processes, the model reveals how different types of vessels work together to maintain stable blood flow across a range of pressures. The researchers identified four distinct phases of vascular response. At very low pressures, blood flow drops below optimal levels. As pressure increases, the system enters a “sweet spot” where flow remains steady and well-regulated. Beyond a certain threshold, however, the vessels lose control and blood flow rises rapidly—potentially stressing or damaging delicate vessel walls.
One of the study’s key findings is that not all vessels contribute equally to maintaining healthy circulation. TZ vessels, in particular, make the most critical adjustments to protect the brain. In the outer layers of the brain, capillary sphincters and precapillary arterioles take the lead in regulating flow. Deeper in the brain, penetrating arterioles become more active. This layered response helps ensure that oxygen and nutrients reach the right areas, even during intense neural activity or shifts in systemic blood pressure.
Lead researcher Dr. Ramin Pashaie emphasized that understanding these mechanisms is essential for developing better diagnostic tools and therapies. The model could be used to simulate how diseases disrupt blood flow or to test how new treatments might restore balance. It also lays the groundwork for smarter brain-computer interfaces and neuroimaging technologies that rely on blood flow signals.
By capturing the complexity of cerebral blood regulation, FAU’s model offers a powerful new lens for studying brain health and disease.
Article from Florida Atlantic University: FAU engineers and sensing institute map the brain’s blood flow
Abstract in PLOS One: Depth-dependent contributions of various vascular zones to cerebral autoregulation and functional hyperemia: An in-silico analysis