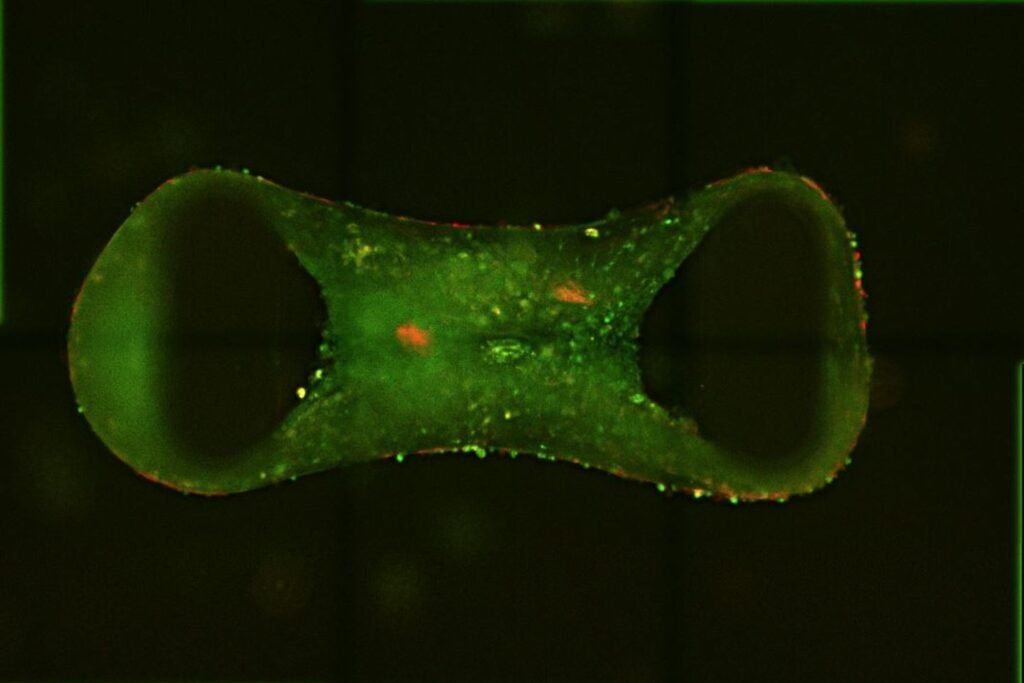
For patients with inherited heart conditions—especially children—treatment options are often limited, and studying the disease in the lab has long been a challenge. That’s because traditional stem-cell-derived heart cells tend to remain immature, behaving more like fetal tissue than adult myocardium. But researchers at QIMR Berghofer Medical Research Institute in Australia have developed a breakthrough solution: lab-grown “tiny hearts” that closely mimic the structure and function of adult human heart muscle.
These miniature cardiac tissues, known as cardiac organoids, are created from human pluripotent stem cells. To overcome the immaturity problem, the team activated two key biological pathways that simulate the effects of exercise, nudging the cells toward a more adult-like state. The result is a three-dimensional, chia-seed-sized organoid that beats, pumps, and responds to drugs like real heart tissue. This maturation step is crucial—it allows researchers to model diseases that typically emerge in adolescence or adulthood with far greater fidelity.
Led by Professor James Hudson, the Cardiac Bioengineering Lab at QIMR Berghofer used these organoids to model several genetic heart diseases, including desmoplakin cardiomyopathy—a condition that’s been notoriously difficult to study. The diseased organoids developed hallmark features of the condition, such as fibrosis (scarring) and reduced contractile function. When treated with a class of experimental drugs known as BET inhibitors, the organoids showed improved pumping ability, suggesting a potential therapeutic path forward.
The project was a collaboration with the Murdoch Children’s Research Institute and The Royal Children’s Hospital, which contributed gene and protein analysis as well as tissue samples from the Melbourne Children’s Heart Tissue Bank. This integration of clinical and lab-based data helped validate the organoids as accurate models of real-world disease. According to Associate Professor Richard Mills, the approach could dramatically accelerate the discovery of new treatments by enabling high-throughput drug screening in human-like tissue.
Beyond drug testing, the technology opens the door to personalized medicine. Because the organoids can be grown from a patient’s own cells, they could one day be used to predict how an individual will respond to a specific therapy—before that therapy is ever administered. That’s especially valuable in pediatric cardiology, where treatment decisions must be made with limited data and high stakes.
While the organoids are still in the research phase, their potential is enormous. They offer a scalable, ethically sound, and biologically relevant platform for studying heart disease in ways that animal models and immature stem cells simply can’t match. And for families affected by rare genetic heart conditions, they represent something even more powerful: a new way forward.
Check out this video that explains more about the cardiac organoids:
Article from QIMR Berghofer: Lab-grown ‘tiny hearts’ bring hope for children and adults with genetic heart disease
Abstract from Nature Cardiovascular Research: Maturation of human cardiac organoids enables complex disease modeling and drug discovery